CAMPBELL, Calif. | PRNewswire
CAMPBELL, Calif., March 22, 2022 /PRNewswire/ -- Vivalink, a leading provider of digital healthcare solutions, is providing its Biometric Data Platform to help detect neutropenic fever events. Neutropenic fever is a common and potentially serious complication of cytotoxic chemotherapy, and early detection and treatment are key to effective management of this condition. As neutropenic fever sometimes occurs without symptoms or during non-waking hours, traditional spot-check methods are limited in their ability to detect some episodes.
In a joint non-randomized study, 80 cancer patients undergoing outpatient intermediate-risk chemotherapy regimens will use Vivalink's continuous temperature monitor, a clinical grade wearable thermometer accurate to within 0.1°C, to record patients' axillary temperature every 10 minutes for the first 84 days of each regimen. If a fever is detected during these incremental checks, both patients and their treating oncologists will be notified in real-time, and patients will get their blood drawn to confirm neutropenia. The study will be led by investigators at UCLA Jonsson Comprehensive Cancer Center.
Another organization developing monitoring solutions for oncology treatments is Alacrity Care, where it's creating clinical-grade digital monitoring solutions that connect patients, providers and caregivers throughout the cancer treatment journey. Using Vivalink's platform for remote continuous temperature and ECG monitoring, Alacrity Care not only offers better insights into what is happening throughout the treatment journey, but also helps to avoid unnecessary hospitalizations due to more proactive care.
"Vivalink's platform ability to produce accurate, medical-grade data has attracted different applications and uses from pharmaceutical to medical technology companies, demonstrating the immense value and need for this data in the healthcare industry," said Jiang Li, CEO of Vivalink.
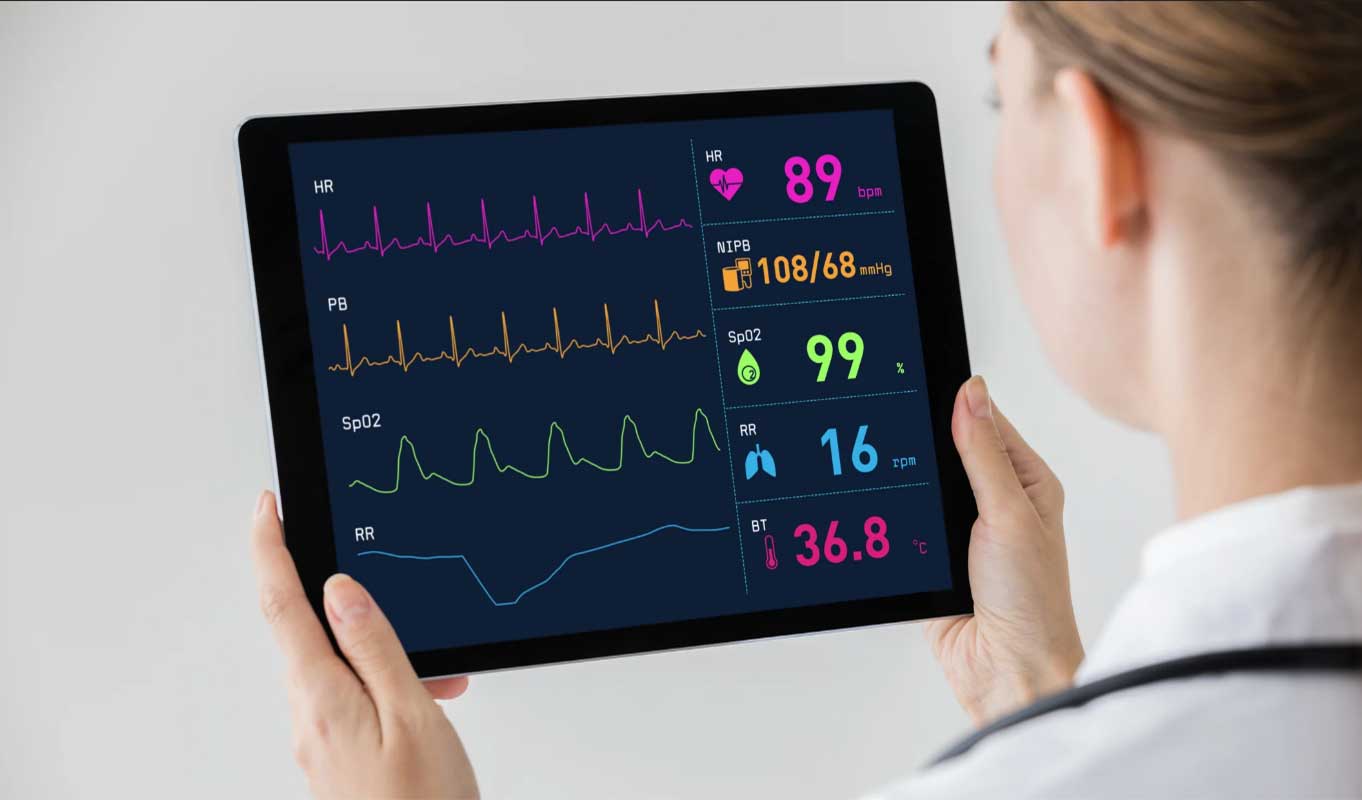
Vivalink's Biometrics Data Platform includes a portfolio of FDA, CE, and NPMA cleared reusable wearable sensors to detect a range of human vitals such as heart rate, respiratory rate, temperature, oxygen saturation, blood pressure and ECG. In addition, the platform includes an FDA and CE cleared arrhythmia detection algorithm, as well as cloud data services that are HIPAA and GDPR compliant.
Vivalink is at the forefront of remote patient monitoring (RPM) and decentralized clinical trials, which have become increasingly common since COVID-19, and its platform has been successfully deployed in a variety of clinical applications around the world.
About Vivalink
Vivalink is a provider of digital healthcare solutions for virtual patient care and decentralized clinical trials. We leverage unique physiology-optimized medical wearable sensors and data services to enable a deeper and more clinical understanding between provider and patient.
Media Contact:
pr@Vivalink.com
VivaLNK, Inc.
51 E. Campbell Avenue, Suite 160
Campbell, California 95008
Sales: info@vivalink.com
Support: support@vivalink.com
No Comments Yet
Let us know what you think